The Uranium-Lead (U-Pb) Dating is a method that encompasses several techniques that are employed in determining the geological age of both terrestrial and extraterrestrial (for example, meteorites) rocks. The method was first suggested by Boltwood in 1907 when he postulated lead to be the decay product of uranium. The method has a dating range from 4.55 x 109 years ago to approximately 5 x 104 years ago, and takes advantage of the long decay chains of the radioactive parent atoms such as 238U, which spontaneously decays through 13 transitional stages until it reaches 206Pb, which is stable and is termed the daughter.
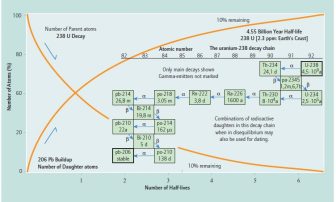
238U has a half-life of 4.47 x 109 years; the transitional stages have an accumulated decay time of 0.0073% of the primary half-life permitting the parent-daughter comparison for chronometric purposes if a closed system with accompanying equilibrium conditions exists. More recently, Uranium-Lead Dating methods based on closed system disequilibrium conditions have been developed (Figure 1).
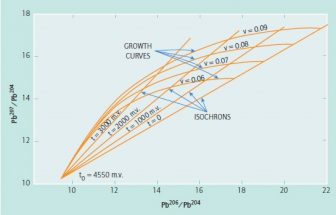
 Lead contains a mixture of four isotopes: 204Pb, which is primordial and stable, and three radiogenic isotopes 208Pb, 207Pb, and 206Pb, which are the end-product daughters of 232Th, 235U, and 238U (232Th: t = 1.41 x 1010yrs; 235U: t = 7.038 x 108 yrs; 238U: t = 4.47 x 109 yrs). Therefore, the ratio (208,207,206pb/204pb) of the respective daughters (9.56, 10.42, 28), to the original 204Pb will decrease with time in a measurable manner termed a “growth curve” that can provide what is called a “model age” using the ratios. The ratio change with time can be seen in the diagram of 207Pb/204Pb vs. 206Pb/204Pb (Figure 2).
Lead contains a mixture of four isotopes: 204Pb, which is primordial and stable, and three radiogenic isotopes 208Pb, 207Pb, and 206Pb, which are the end-product daughters of 232Th, 235U, and 238U (232Th: t = 1.41 x 1010yrs; 235U: t = 7.038 x 108 yrs; 238U: t = 4.47 x 109 yrs). Therefore, the ratio (208,207,206pb/204pb) of the respective daughters (9.56, 10.42, 28), to the original 204Pb will decrease with time in a measurable manner termed a “growth curve” that can provide what is called a “model age” using the ratios. The ratio change with time can be seen in the diagram of 207Pb/204Pb vs. 206Pb/204Pb (Figure 2).
Furthermore, there are three diagrams that can be generated from rock data that provide ages from the straight lines produced by plotting 206Pb/204Pb vs. 238U/204Pb, 207Pb/204Pb vs. 235U/204Pb, and 208Pb/204Pb vs. 232Th/204Pb. Therefore, an independent number of independent estimates of the rock’s age can be obtained by measuring the lead isotopes and their parent isotopes. If the results agree, it is likely the age obtained is a fair approximation of the actual age. If the results conflict—a common problem because uranium and lead may be gained or lost from many minerals—the problem of loss or gain of isotopes is readily apparent. Using plots such as that shown in Figure 2 (lead-lead technique), the effects of lead loss can be minimized because it determines the ages from the lead isotopes alone. This approach permits a researcher to chart at what points in time metamorphic heating events may have occurred, thus helping to unravel the geological history of a rock or mineral.
Figure 1 shows both the radioactive parent-stable daughter relationship between 238U and 206Pb, and some radioactive parent-radioactive daughter relationships. The latter relationships provide dating potential for closed systems in disequilibrium, while the former provide dating potential in closed systems in equilibrium.
Figure 2 shows the important role that ratios of lead isotopes play in dating minerals. Growth curves can be determined independently of the uranium content. V= 235U/204Pb.
 With the development of more sophisticated analytical techniques in recent decades for directly measuring parent and daughters and their a, P and y decay products, attempts have been made to quantify in a meaningful way the intermediate decay products of 232Th, 235U, and 238U. These decay products are compared both with the parents and among themselves producing a wide rage of techniques that span a period of days to greater than a million years (234U/238U: 50 x 103-1.5 x 106 yrs; 227Th/235U: 10-200 x 103 yrs; 224Ra/228Ra: 2-20 yrs, 210Bi/210Pb: hours -50 days).
With the development of more sophisticated analytical techniques in recent decades for directly measuring parent and daughters and their a, P and y decay products, attempts have been made to quantify in a meaningful way the intermediate decay products of 232Th, 235U, and 238U. These decay products are compared both with the parents and among themselves producing a wide rage of techniques that span a period of days to greater than a million years (234U/238U: 50 x 103-1.5 x 106 yrs; 227Th/235U: 10-200 x 103 yrs; 224Ra/228Ra: 2-20 yrs, 210Bi/210Pb: hours -50 days).
They are based on the disequilibrium of a daughter decay product rather than the equilibrium required for dating using the parent and stable daughter. These methods require that either a parent or a daughter be chemically separated by natural processes and remain thereafter in a closed system. The age limits obtainable are based on the half-life of the daughter or daughters, and the age upon the measured degree of return to equilibrium conditions. Unlike the rock and mineral limitation of traditional Uranium-Lead Dating, the disequilibrium method can be used on a wide variety of materials, including minerals in young volcanic rocks, bones, egg shell, wood, mollusks, terrestrial carbonates, and marine and lacustrine sediments.
References:
- Farquhar, R. M. (2003). Lead isotopes in archaeology. In: Physics and Archaeometry. Special issue of Physics in Canada, 59(5), 275-284.
- Russell, R. D., & Farquhar, R. M. (1960). Lead isotopes in geology. New York: InterScience Publishers.
- York, D., & Farquhar, R. M. (1973). The earth’s age and geochronology. Toronto: Pergamon Press.

