Potassium argon [K/AR] dating is a radiometric technique that can be applied throughout the time span of the Earth from 4.5 billion years ago down to a few tens of thousands of years ago. Thus, K/Ar is the method that has been most useful in unraveling the history of the earth, the development of life, and most importantly, the history of human development. The method is based on the properties of potassium, which is one of the most abundant elements of the continental Earth’s crust comprising 2.4 weight percent. There are three naturally occurring isotopes of the element potassium: 39K (93.258%), 40K (0.0117%), and 41K (6.73%). The dating technique is based on the radioactive decay of the isotope 40K, which has a half-life of 1.28 x 109 years. Primordial 40K, which at formation would have had an abundance of about 14%, has diminished by a factor of over a thousand in the 10 half-lives since the Big Bang approximately 13 billion years ago, and over a factor 10 because the three half-lives that have occurred since the origin of life on Earth 3.8 billion years ago.
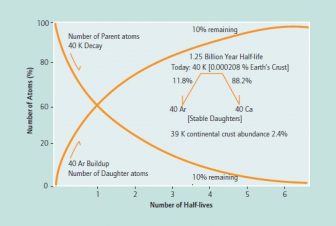
Unlike the radiocarbon dating, where the amount of the cosmogenically produced radioactive carbon isotope remaining need only be compared to its original concentration, K/Ar dating determinations are based on the binary decay of 40K to either 40Ca (88.2%) or 40Ar (11.8%). It is the steady buildup of the daughter isotope 40Ar that represents the atomic clock and its ticking. The clock starts when a molten rock cools and solidifies, having liberated any former 40Ar, and is read by comparing the amount of 40K present when formed to the amount of 40Ar present (see figure). This comparison is possible because the ratio of 39K to the decaying 40K is known, and 39K is the most abundant stable isotope that can be measured. This technique requires that the 40K, 39K and 40Ar, 36Ar be analyzed separately to produce the data required to calculate an age. The obvious complication of this technique is the fact that 40Ar is an inert gas that makes up 1% of the atmosphere and can diffuse into samples biasing the calculated results. Furthermore, the gas can escape from samples, thus producing apparent younger ages. The modern ratio of 40Ar to 36Ar is 295.5, which permits the analyst to subtract the atmospheric 40Ar from the radiogenic 40Ar in a sample to arrive at the amount produced by 40K decay.
 A new approach has been adopted in recent years in which the 40Ar and 40K are measured simultaneously by irradiating the sample and converting some of the 39K into 39Ar, which does not occur naturally. The 39Ar half-life of 269 years is long enough to cause no problems in the age calculations. This approach permits the 39K and the 40K to be directly determined along with the 40Ar and eliminating the need to separately analyze for potassium. The addition of lasers in
A new approach has been adopted in recent years in which the 40Ar and 40K are measured simultaneously by irradiating the sample and converting some of the 39K into 39Ar, which does not occur naturally. The 39Ar half-life of 269 years is long enough to cause no problems in the age calculations. This approach permits the 39K and the 40K to be directly determined along with the 40Ar and eliminating the need to separately analyze for potassium. The addition of lasers in
place of induction heating technology has permitted more precise analyses to be made. This approach permits the analyses of single grains, and the use of a more refined step-heating approach that greatly improves the reliability of the result and eliminates the need to do an independent analysis for the potassium.
The figure shows the parent-daughter relationship between the radioactive 40K and the stable 40Ar. Only 11.8% of 40K atoms decay to 40Ar.
References:
- Dalrymple, G. B., & Lanphere, M. A. (1969). Potassium argon dating. San Francisco, CA:
- H. Freeman. Smith, P. (2003). 40K-40Ar Dating in Archaeology. In L. A. Pavlish & A. E. Litherland (Eds.), “Physics and Archaeometry.” Special issue of Physics in Canada, 59(5), 257-262.
- York, D., & Farquhar, R. M. (1973). The Earth’s age and geochronology. Toronto: Pergamon Press.

