Radiocarbon is the best-known radiometric dating technique due to its successful application to problems in human history and prehistory for over 50 years.
Willard Libby’s development of the technique in the late 1940s permitted relative time to be sorted radio-metrically in archaeological contexts in a manner that eclipsed the more traditional relative dating methods that had developed over the previous century.
Carbon-14 is a radioactive isotope produced in the upper atmosphere when cosmic rays generate neutrons as they bombard that outer atmosphere. These neutrons can enter abundant nitrogen-14 nuclei (78% of the atmosphere), converting some of them to carbon-14. The carbon-14 combines with oxygen to form carbon-14 dioxide, which is assimilated by plants and the animals that consume those plants. The carbon-14 production is relatively constant over short periods of time and is in equilibrium with the carbon sinks in the environment (see figure).
The Libby radiometric clock is started when the organic item (animal or plant) dies. The clock is set to “0” at death and begins to tick, with a half-life of 5,568 years. After 5,568 years, one half of the original radio-carbon-14 remains; after 2 x 5568 years, one quarter is left, and so on. There are two methods employed today to determine the amount of radiocarbon remaining in a sample: (1) decay counting, the traditional method, which relies on the decay of carbon-14 to nitrogen-14 with the emission of a beta ray that can be measured in decays per minute per gram and compared with a standard that has a decay rate of 15 decays per minute per gram; and (2) accelerator mass spectrometry (AMS), the atom-counting method, which counts the atoms of radiocarbon-14 in a sample and compares that result with those of a standard.
Libby made a fundamental assumption when he developed his technique. He assumed that the rate of carbon-14 production was constant through time. Subsequently, this assumption was shown to be untrue, but the difficulty could be overcome by instituting a calibration that compared the radiocarbon age of an item with the radiocarbon age of a wood sample of known age. Today this calibration curve is known as the dendrochronological correction curve, and it allows the dated sample’s age to be expressed either in calendrical years B.c.(or B.C.E.) or A.D.(or C.E.), or as a conventional radiocarbon-14 age expressed as B.P. (before 1950 in radiocarbon years). Today, there is a convention, not universally accepted, that radiocar-bon-14 dates that have not been dendro-corrected (for example, very old dates [> 40,000], groundwater dates, or atmospheric pollution dates) should be designated with a lowercase BP (before present). AMS does not escape this dendro-correction limitation, but does offer advantages in that smaller samples can be used (milligrams vs. grams); shorter counting times are possible due to improved counting statistics (hours vs. days); the backgrounds associated with cosmic rays, which must be shielded against in the traditional method, are not a factor; and ages up to 70,000 years are measurable because of lower backgrounds and greater counting statistics.
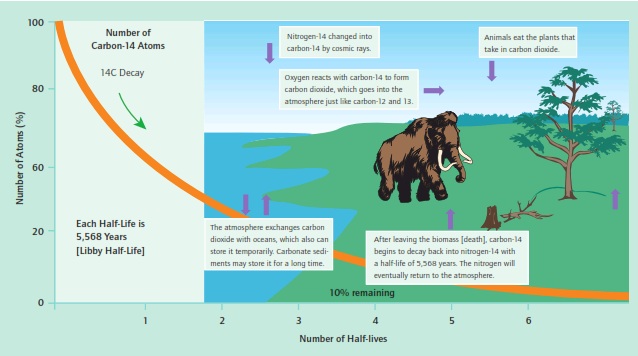 Tab.1 – The decay of carbon-14 through 6.64 half-lives or 37,000 years, after which only 1% of the original carbon-14 remains. The production, mixing, and fate of the carbon-14 as part of the carbon cycle is shown in the insert.
Tab.1 – The decay of carbon-14 through 6.64 half-lives or 37,000 years, after which only 1% of the original carbon-14 remains. The production, mixing, and fate of the carbon-14 as part of the carbon cycle is shown in the insert.
A further complication in some kinds of materials used for radiocarbon dating is the issue of the natural introduction of old carbon into living organisms in and around oceans, due to the presence of old carbon from carbonate sources and upwelling water. A marine correction can be made for this effect in parts of the world where the offset is known. The standard method for determining this offset is to date a sample that was collected prior to 1950 (prior to the atomic bomb testing peak) and determine the difference between actual age and the radiocarbon age.
The two analytical methods exist side by side today, each having a specific market niche. While AMS may be preferred when samples are small or high precision dates (errors of less than 60 years) are required, the traditional beta decay method provides, at a reasonable price, perfectly acceptable results when sample size or high precision are not an issue. Not all laboratories are equal throughout the world, and caveat emptor should be kept in mind when choosing a lab from which to obtain results, for there exist those that are good, bad, and ugly. Surprisingly, this state of affairs has more to do with the chemistry of sample preparation, which varies greatly from lab to lab, than it does with either the AMS or decay systems themselves. There are slightly fewer than 100 active traditional radiocarbon dating labs throughout the world and more than two dozen AMS labs.
References:
- Bennett, C. L., Beukens, R. P., Clover, M. R., Elmore, D., Gove, H. E., Kilius, L., et al. (1978). Radiocarbon dating with electrostatic accelerators: Dating of milligram samples. Science, 201, 345-347.
- Libby, W. F. (1955). Radiocarbon dating. Chicago: University of Chicago Press.
- Pavlish, L. A., & Banning, E. B. (1980). Revolutionary developments in carbon-14 dating. AmericanAntiquity, 45(2), 290-296.

